Successful shared audits will need mindful preparing, potent high quality devices proper documentation and proactive customer service.
Hazard management emphasis: With all-natural dangers discovered as an important chance to supply chain integrity, guaranteeing stable storage environments by means of State-of-the-art temperature monitoring can mitigate possibility exposures.
Are all production batch documents and launch take a look at outcomes initial reviewed for completeness and accuracy in advance of the release of a batch of concluded merchandise?
You have to validate that proper statistical approaches are employed (in which necessary) to detect recurring good quality challenges
Our workforce of senior GMP auditors, who can be found all over the world, have in depth knowledge of conducting pharma audits to GMP, familiarity with the pharmaceutical regulatory specifications, expectations and treatments.
SimplerQMS employs the knowledge you supply to us to Get in touch with you about our related content material, and solution details. You could unsubscribe from these communications Anytime. To learn more, see our Privateness Policy.
High quality audits are systematic examinations to ascertain if pursuits adjust to options and restrictions. Pharmaceutical suppliers use audits to confirm compliance with Excellent Manufacturing Practices (GMP). Audits have two objectives - to validate production systems are managed and to allow timely issue correction. Audits Examine GMP compliance in output and high-quality Regulate.
By way of example, This might include things like auditing the purity with the products and solutions developed through the R&D Division constitutes a tier two example.
To be a consequence, you should have an obligation to interact with the progressively advanced supply chain, and here all source chain actors which include a multitude of suppliers, service companies and subcontractors.
The maker is likewise responsible for processing the issues immediately, documenting criticism evaluations and investigations as well as sharing info across pertinent departments and regulatory bodies.
This document offers an introduction to auditing along with the audit procedure. It defines an audit as being the on-website verification of the procedure or excellent program to ensure compliance. Audits is usually done internally or externally In read more line with ICH guidelines. The goals of the audit are to ascertain conformity or nonconformity with high quality units and to improve excellent.
Check out the transformative part of Synthetic Intelligence in pharmaceutical exploration by way of insightful interviews with industry professionals. Explore how AI is revolutionizing drug discovery, driving precision…
This phase identifies and establishes the foundation explanation for the trouble by investigating the readily available knowledge. The necessary facts must be accurately depicted and preserved to reflect the actual cause of the trouble.
The GMP audit checklist is a comprehensive listing of items that need to be reviewed for the duration of a GMP audit. The WHO has posted a GMP audit checklist that covers all components of pharmaceutical manufacturing. The subsequent are many of the products A part of the checklist:
 Ben Savage Then & Now!
Ben Savage Then & Now!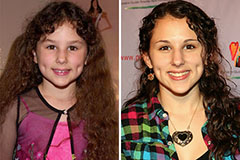 Hallie Eisenberg Then & Now!
Hallie Eisenberg Then & Now! Hailie Jade Scott Mathers Then & Now!
Hailie Jade Scott Mathers Then & Now!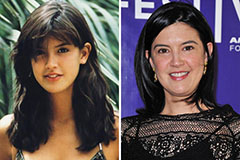 Phoebe Cates Then & Now!
Phoebe Cates Then & Now! Atticus Shaffer Then & Now!
Atticus Shaffer Then & Now!